Abstract
INTRODUCTION: Graduate medical education (GME) training in hematology in the United States is accredited by the Accreditation Council for Graduate Medical Education (ACGME). Trainees provide patient care that requires manual review and interpretation of peripheral blood smears (PBS). The ACGME milestones were developed with significant input from the American Society of Hematology (ASH) and mandate fellow competency in the interpretation of peripheral blood smears. However, there are currently no publications that define competency or propose consensus recommendations (CRs) for PBS review. The primary aim of this study was to establish CRs on PBS review with a focus at the GME level.
METHODS: Nominal group technique (NGT) and constructivist grounded theory (CGT) were selected given the lack of existing published theory and consensus on the methodology of PBS review or other analogous visuospatial skills within GME. A convenience sample of hematologists with expertise in training and assessing fellows was recruited through the ASH Medical Educators Institute. Goal accrual was 5-10 hematologists. Participants were required to regularly review PBSs as part of their clinical practice and attend two 1-hour virtual focus group sessions. The facilitator guide for session 1 was developed a priori to prompt open discussion on PBS review. Dialogue was transcribed and organized into thematic concepts through inductive coding. Data from session 1 were analyzed using a mixed methods approach and were used to develop a facilitator guide for session 2. Draft CRs were created using CGT. Weak and strong consensus were pre-specified as 50 - 69%, and >70% agreement, respectively. CRs underwent iterative review which would continue for a pre-specified minimum of 2, but no more than 3 rounds.
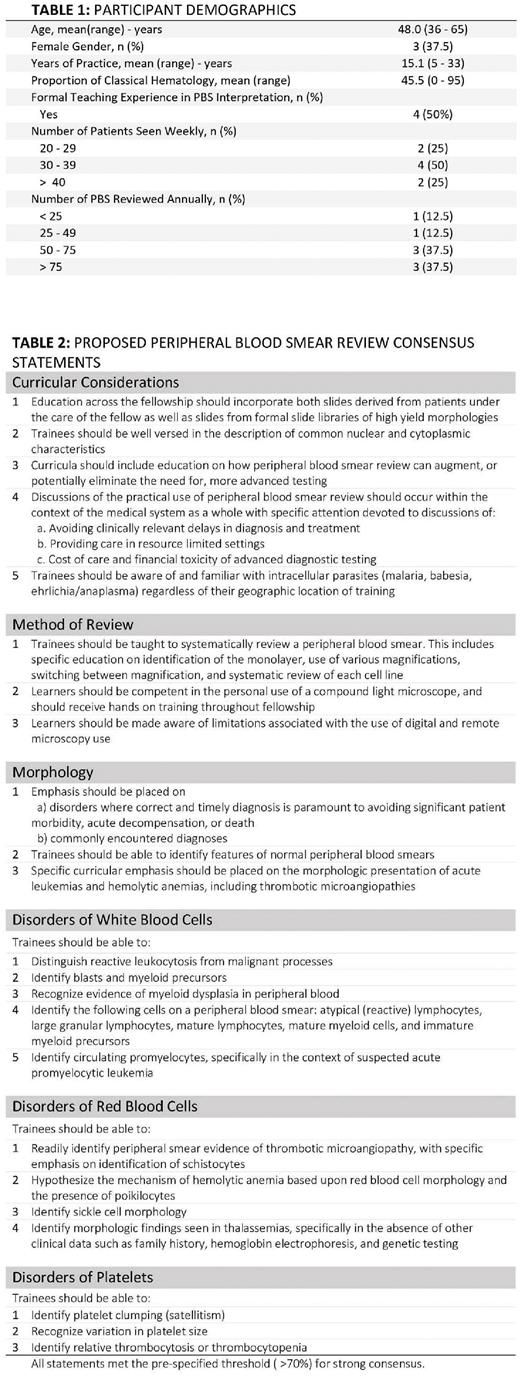
RESULTS: Eight academic hematologists (5 men, 3 women) enrolled in the focus group (Table 1). Duration of clinical practice ranged from 5 to 33 years. Clinical focus spanned classical and malignant hematologic conditions. Half had formally instructed trainees in course work on PBS morphology. All routinely worked with fellows.
CRs were generated through CGT. Three rounds of review occurred. Participants provided a statement of agree vs. disagree and offered qualitative suggestions for each statement in which they disagreed. Response rates per round were 87.5%, 87.5%, and 100%. The most commonly identified themes included recognition of morphology suggestive of thrombotic microangiopathies and distinguishing between malignant vs. benign white blood cells (WBC), specifically as it pertains to acute leukemias.
Strong consensus was reached on multiple aspects of PBS education, including curricular considerations, method of review, and morphology (Table 2). All agreed that trainees should learn PBS review through a systematic approach, although some agreed that this systematic approach is not always used in clinical practice. The group agreed that trainees should be educated through PBS review of patients under their clinical care as well as through formal slide "libraries". Many discussed the changing landscape of PBS education with the advent of digital microscopy, and formed strong consensus that trainees should understand the benefits and limitations of digital microscopy.
Group discussion largely focused on disorders of WBCs and red blood cells (RBC) (43% and 48% of all coded statements respectively). Most discussion focused on disorders in which prompt recognition was imperative to avert significant patient morbidity or mortality. However, all agreed that trainees must also be competent in the identification of common conditions, regardless of the degree of clinical importance, including normal peripheral blood smear morphology. The group was unable to reach consensus on how to balance these competing philosophies.
CONCLUSIONS: A formal theory of PBS education was systematically developed using CGT. Experts were able to form strong CRs on almost every aspect of PBS review at the GME level.
FUTURE DIRECTIONS: We propose that these guidelines serve as a template for future research and curricular development for both PBS review and other visuospatial tasks in medical education. This framework can also be used by organizations, such as ACGME and ASH, when formally reviewing and codifying guidelines on PBS review.
Disclosures
Reid:Xencor: Research Funding; Millennium: Research Funding; Aptose: Research Funding; ADC Therapeutics: Research Funding; Epicentrx: Other: Spouse is employed by epicentrx. Gerds:Accurate Pharmaceuticals: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Research Funding; Imago BioSciences: Research Funding; Kratos Pharmaceuticals: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hobbs:Keros: Other: Advisor or review panel participant; Pharmaxis: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pfizer: Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Bayer: Research Funding; Merck: Research Funding. Zumberg:Veralox Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal